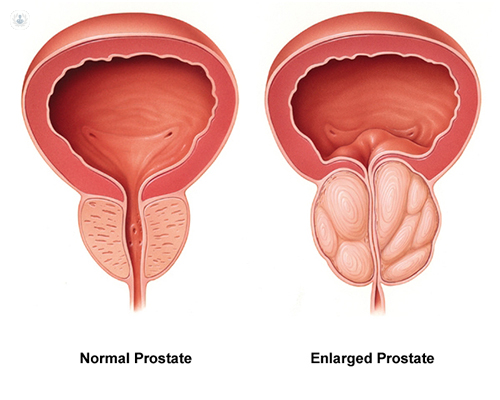
Aside from non-melanoma skin cancer, prostate cancer is the most common cancer among men in the United States. It is also one of the leading causes of cancer death among men of all races and Hispanic origin populations.(1)
Dietary Boron intake is inversely correlated with prostate cancer incidence. (5)
Prostate risk is 52% lower in men whose diets contain at least 1.8mg boron daily. Prostate cancer risk reduction is correlated with boron intake. Recommended optimal daily dose of elemental boron is 3 mg/day for health maintenance.
Mechanisms of Action
Boron-containing compounds interfere with the physiology and reproduction of cancer cells through diverse mechanisms, including inhibition of serine proteases, NAD-dehydrogenases, mRNA splicing and cell division, receptor binding mimicry, and induction of apoptosis. (2)
In several studies Barranco et al (7, 8, 9, 10) demonstrate that increased boron intake is associated with lower levels of Prostate Specific Antigen (a biomarker for prostatitis and prostate cancer) as well as modulation of serum estradiol leading to increased expression of ER-beta receptors and decrease of ER-alpha receptors on tumor cells decreasing proliferation and growth signaling. Furthermore boron supplementation increased regulation of cell cycle arrest, increased apoptosis, decreased cell adhesion, migration and metastatic progression.
Boron decreases cancer associated inflammatory factors including hs CRP, TNFa, Interleukin-6 all of which are elevated in the tumor microenvironment. “Elevated hs-CRP is associated with an increased risk for breast cancer, obesity and metabolic syndrome (MetS) in children, atherosclerosis, unstable angina, insulin resistance, type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), metastatic prostate cancer, lung cancer, adult depression, depression in childhood and psychosis in young adult life, coronary heart disease, and stroke." (2)
In murine study Insulin Like Growth Factor was significantly reduced in the prostate tumor cells in boron dosed animals. The IGF-1 signaling pathway promotes cancer progression and its down regulation is associated with lower risk of prostate cancer. (6)
“Increased intracellular concentration of borate activates borate transporters and leads to growth inhibition and increased apoptosis. “ (2) (3) (11)
Boron may act as a Histone De-acetylase Inhibitor (HDI). HDI’s act as therapeutic agents for cancer due to their impact on gene expression on growth arrest signaling, cell differentiation and apoptosis in cancer cells. (2)
Boronic Acid has been shown to inhibit hypoxia inducible factor (HIF) which is a physiological stimulus for tumor induced angiogenesis. Inhibition of HIF leads to inhibition of Vascular Endothelial Growth Factor (VEGF) and the production of capillary blood supply to tumor cells. Angiogenesis leads to exponential tumor growth and to metastatic progression of solid tumors. (11)
Plant foods rich in boron include Avocados, dried apricots, dried prunes, raisins, red kidney beans, lentils, almonds, hazelnuts, brazil nuts, pistachios, cashews and walnuts.
In summary, dietary and supplemental oral boron intake should be optimized to create a tumor microenvironment and cancer terrain that decreases risk of prostate carcinogenesis,proliferation and disease progression through angiogenesis and metastasis.
Selected References
1. American Cancer Society Statistics https://cancerstatisticscenter.cancer.org/
2. Scorei RI, Popa R Jr. Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anticancer Agents Med Chem. 2010 May;10(4):346-51. doi: 10.2174/187152010791162289. PMID: 19912103.
3. Romulus Ion Scorei and Radu Popa, Sugar-Borate Esters –Potential Chemical Agents in Prostate Cancer Chemoprevention DOI: 10.2174/18715206113139990124 Volume 13, Issue 6, 2013 Page: [901 - 909]
4. Kiliccioglu I, Konac E, Varol N, Gurocak S, Yucel Bilen C. Apoptotic effects of proteasome and histone deacetylase inhibitors in prostate cancer cell lines. Genet Mol Res. 2014 May 9;13(2):3721-31. doi: 10.4238/2014.May.9.17. PMID: 24854658.
5. Cui Y, Winton MI, Zhang ZF, et al. Dietary boron intake and prostate cancer risk. Oncol Rep. 2004;11(4):887-892.
6. Pizzorno, L. Review: Nothing boring about boron. Integrative Medicine Vol. 14, No. 4 August 2015
7. Barranco WT, Hudak PF, Eckhert CD. Evaluation of ecological and in vitro
effects of boron on prostate cancer risk (United States). Cancer Causes Control. 2007;18(1):71-77.
8. Barranco WT, Eckhert CD. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett. 2004;216(1):21-29.
9. Barranco WT, Eckhert CD. Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br J Cancer. 2006;94(6):884-890.
10. Barranco WT, Kim DH, Stella SL Jr, Eckhert CD. Boric acid inhibits stored Ca2+ release in DU-145 prostate cancer cells. Cell Biol Toxicol. 2009;25(4):309-320.
11. Shimizu K, Maruyama M, Yasui Y, Minegishi H, Ban HS, Nakamura H. Boron-containing phenoxyacetanilide derivatives as hypoxia-inducible factor (HIF)-1alpha inhibitors. Bioorg Med Chem Lett. 2010;20(4):1453-1456.