Today, more and more patients are avoiding toxic chemotherapy in favor of targeted cancer therapies. Among the many new therapies available are a class of immunotherapy drugs that take the brakes off of the immune system and mobilize T cells against tumor cells.
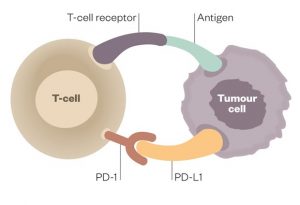 Because tumor cells have the capacity to disable T cells, this therapy addresses the huge problem of immune resistance in many cancers. Drugs in the class of PD1 and PDL1 inhibitors were some of the first to be developed. These drugs bind to PD1 or PDL1 receptors on the tumor surface and unleash the fury of the immune system upon the tumors by removing the inhibitory function of these ligands.
Because tumor cells have the capacity to disable T cells, this therapy addresses the huge problem of immune resistance in many cancers. Drugs in the class of PD1 and PDL1 inhibitors were some of the first to be developed. These drugs bind to PD1 or PDL1 receptors on the tumor surface and unleash the fury of the immune system upon the tumors by removing the inhibitory function of these ligands.
Nature has designed the immune system with both a gas pedal and a brake. The PD1 and PDL1 inhibitors are the brakes. Take off the brakes and the immune system is activated.
The best of outcomes with these treatments may result in complete tumor eradication, a truly miraculous outcome for some patients. I have a patient who came to me with Stage 4 Endometrial Cancer with Lung Metastases some years ago. After reduction of some of her tumor burden with surgery and rather brutal chemotherapy, her very forward-thinking Gynecologic Oncologist included a course of Keytruda (Pembrolizumab), which was a new immunotherapy treatment at the time.
The historical prognosis for this patient would have been certain eventual mortality for her metastasized aggressive cancer. However, she achieved a complete response and has been in remission and designated NED or No Evidence of Disease for many years now. This is a patient who most likely would not be alive today without the advent of PD1 -PDL-1 inhibitor therapy.
The problem with this class of drugs is that their use is very unskillful and very unpredictable. Some patients will respond with a modicum of mild to moderate systemic autoimmune inflammation while other patients will be disabled by furious, extreme, and damaging autoimmune syndromes. Some patients may die from extreme autoimmune activity. I had one patient who developed myocarditis and died within a few days of receiving his first dose. This was a prostate cancer patient whose sudden death was completely unexpected and not predicted.
These patients require a health model and safe, effective modulation of extreme auto-immune inflammation not provided by their oncology teams.
Some patients will have immune activation similar to a nice warm burning ember. They get the therapeutic benefit without extreme adverse effects. While other patients will respond with a forest fire of inflammation that must be suppressed aggressively with steroid medications for long periods of time. The adverse effects of long-term steroid therapy then become part of the clinical picture and challenge for these patients. In these circumstances, it IS reasonable to ask if the cure is worse than the disease itself?
My patient developed such severe colitis (a common adverse effect) that she visited the emergency room multiple times for fluid and electrolyte replacement due to extreme persistent watery diarrhea. Additionally, the nutritional status of this patient was also compromised and she became depleted in both calories and nutrients and developed sarcopenia.
Many cancer patients receiving PD1 and PDL 1 inhibitors are left with lifelong autoimmune disease. Most common are autoimmune arthritis, colitis, thyroiditis, dermatitis, pneumonitis, and associated loss of normal tissue and organ function. Some patients suffer ongoing chronic inflammatory pain syndromes.
Less prevalent, but also part of the long list of adverse effects are myocarditis, pericarditis, nephritis, hepatitis, pancreatitis, neuritis, vasculitis. Essentially, any tissue or organ can be impacted with associated loss of function and sequelae.
 It is my practice to screen and monitor patients receiving cancer immunotherapies for the development of autoimmune syndromes and intervene early. If I have a patient with a history of inflammatory or autoimmune disorders I can predict that such patients are more likely to develop adverse effects.
It is my practice to screen and monitor patients receiving cancer immunotherapies for the development of autoimmune syndromes and intervene early. If I have a patient with a history of inflammatory or autoimmune disorders I can predict that such patients are more likely to develop adverse effects.
Additionally, high levels of inflammation not only lead to pain syndromes but are also contributors to ongoing chronic fatigue as well as agitation, cognitive changes, sleep disruption, anxiety, and depression, and the stress of living not only as a cancer survivor but with a chronic and distressing autoimmune syndrome difficult to control and manage. It is my firm goal that Quality of Life must be a goal in all treatment plans for cancer patients and survivors.
If a patient has NO inflammatory adverse effects it is assumed that the patient is not going to benefit from the PD1/PDL1 inhibitor because there is no sign or symptom indicating immune activation. I always tell patients we should celebrate if they develop a rash or diarrhea because we know the drug is working!
In fact, it is my observation over many years of following patients who have received these therapies that when the course of immunotherapy treatment is completed those patients who continue to have low levels of inflammation continue to have the therapeutic benefit of tumor control. This is only an empirical observation on my part. For example, the endometrial cancer patient described above continues to have mild colitis and has remained in remission. Before the availability of these therapies, we would expect this patient to have a recurrence and to die of her advanced stage 4 metastatic disease within a few years of her diagnosis. Patients such as this with lung metastases historically had very poor prognoses and very high mortality rates. Patients with powerful and manageable responses to PD1 and PDL1 inhibitors may live a long time. While some patients do recur, some have not. We have not had decades of time to follow these patients as these treatments are relatively new. If nothing else, these treatments do extend the life of many patients.
How can we modulate the auto-immune adverse effects of these potentially curative immunotherapy treatments? I have taken the approach that we employ in Functional and Naturopathic Medicine in the management of auto-immune syndromes to turn down the volume on the immune inflammation just enough to reduce extreme side effects, damage, and loss of function without losing the therapeutic benefit of these immunotherapy treatments.
While we can rely on studies that have demonstrated that Omega 3 Fatty Acids, Vitamin D3, and Curcumin and a healthy microbiome can modulate auto-immune inflammation, there is a paucity of research on managing autoimmune syndromes related to immunotherapy adverse effects with the exception of steroids. (See selected references below.)
I share with you here my empirical clinical experience. I have employed this approach with several hundred patients since immunotherapies have come into wider use in oncology. Clinicians experienced in managing autoimmune syndromes will recognize the basic principles of care.
- Anti-Inflammatory, Low Antigen Diet
- Support for the healthy intestinal microbiome
- Specific Nutriceutical-Phytochemical Interventions
- Omega 3 Fatty Acids (EPA DHA) recommended dose 4-6 grams daily SPMs Specialized ProResolving Mediators can also be considered 1-2grams daily
- Fat soluble Curcumin recommended dose 2-6 grams daily
- Vitamin D3 5,000-25,000iu daily (125mcg-625mcg).
- Consider a loading dose of 50,000iu (1.25mg)
I always start at the lower end of the dose range and spread the dosing out into 3-4 doses over the day. The goal is to MODULATE but not SUPPRESS the therapeutic impact. It is also important to be mindful of the anticoagulant/platelet aggregation inhibitory effect of such an approach and to determine which patients may NOT be a candidate for high dosing due to thrombocytopenia or anticoagulant pharmaceutical therapies.
This approach has few negative drug-nutrient interactions. I have continued these inflammation-modulating therapies continuously for many years with most patients. Dosing is highly individualized to each patient towards the goal of supporting and promoting healthy function and quality of life.
For front-line clinicians interested in supporting the health of cancer patients and cancer survivors and learning and implementing my OutSmart Cancer® System developed over 35 years in practice, I encourage you to join our training program, Foundations of Integrative Oncology, self-paced online training with clinical supervision and mentoring. Go to aiiore.com.
There is a huge population of patients whose lives have been touched by cancer searching for a health model and skilled and knowledgeable clinicians.
Selected References:
Vitamin D and autoimmune diseases.
Illescas-Montes R, Melguizo-Rodríguez L, et al Life Sci. 2019 Sep 15;233:116744. doi: 10.1016/j.lfs.2019.116744. Epub 2019 Aug 8. PMID: 31401314
Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis.
Grover S, Dougan M, et al Cancer. 2020 Aug 15;126(16):3758-3767. doi: 10.1002/cncr.32966. Epub 2020 Jun 22. PMID: 32567084
Therapeutic Potential of omega-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases.
Li X, Bi X, Wang S, Zhang Z, Li F, Zhao AZ.
Front Immunol. 2019 Sep 27;10:2241. doi: 10.3389/fimmu.2019.02241. eCollection 2019. PMID: 31611873
Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases.
Abdolmaleki F, Kovanen PT, et al
Clin Rev Allergy Immunol. 2020 Feb;58(1):82-91. doi: 10.1007/s12016-019-08754-9. PMID: 31267470
Curcumin in Autoimmune and Rheumatic Diseases.
Yang M, Akbar U, Mohan C.
Nutrients. 2019 May 2;11(5):1004. doi: 10.3390/nu11051004.
PMID: 31052496
Curcumin and autoimmune disease.
Bright JJ.
Adv Exp Med Biol. 2007;595:425-51. doi: 10.1007/978-0-387-46401-5_19. PMID: 17569223
Curcumin as an Adjuvant to Cancer Immunotherapy.
Paul S, Sa G.
Front Oncol. 2021 Aug 16;11:675923. doi: 10.3389/fonc.2021.675923. eCollection 2021.
PMID: 34485117
Gut Bacteria Influence Effectiveness of a Type of Immunotherapy. https://www.cancer.gov/news-events/cancer-currents-blog/2018/gut-bacteria-checkpoint-inhibitors. Feb 2018 NCI Staff