Understanding and Monitoring Risk Factors
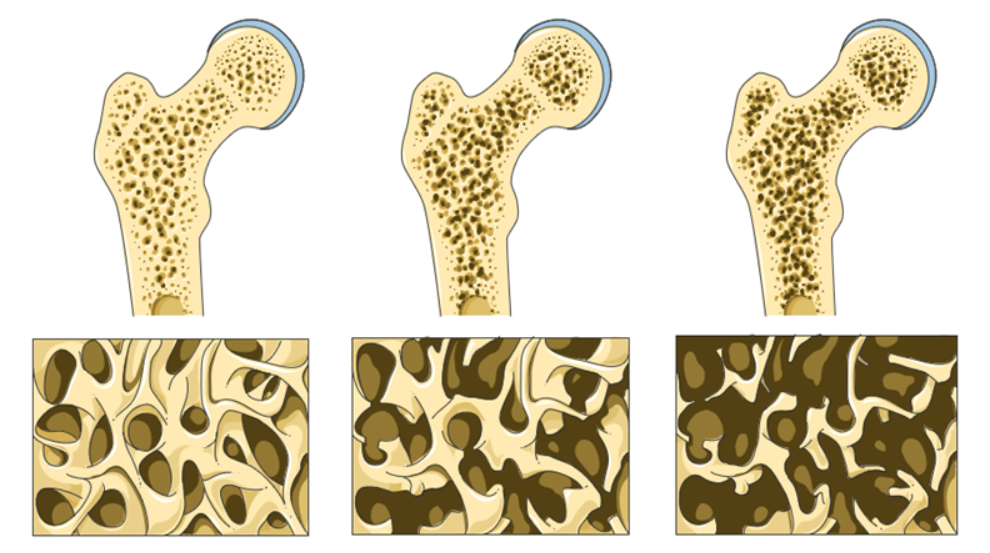
Bone density, or bone mineral density ( BMD ), is the amount of bone mineral in bone tissue. Bone mineral density (BMD) is a lifetime marker of estrogen exposure in a woman's body and has been associated with increased breast cancer risk. Estrogen is a crucial factor in maintaining bone density and gradually decreases over age. While there are many factors that influence bone density and bone health, the presence of estrogen contributes to the capacity of bone to continuously remodel and maintain the dynamic balance between bone resorption and bone formation. A woman’s exposure to estrogen over the life cycle may contribute to her risk of breast cancer.
Bone density measurement is used in clinical medicine as an indirect indicator of osteoporosis and fracture risk. There is a clear association between poor bone density and a higher probability of fracture. There is a clear association between poor bone density and low estrogen levels. Conversely, there is a clear association between increased and healthy bone density and higher estrogen levels.
 Screening for risk of breast cancer should ALSO include assessment of estrogen levels and bone density along with well-recognized risk factors which include first degree relatives, obesity, increased visceral fat, smoking, alcoholism, early menarche, late menopause, sedentary lifestyle, hormone replacement therapy, and prolonged estrogen exposure, increased density of breast tissue.
Screening for risk of breast cancer should ALSO include assessment of estrogen levels and bone density along with well-recognized risk factors which include first degree relatives, obesity, increased visceral fat, smoking, alcoholism, early menarche, late menopause, sedentary lifestyle, hormone replacement therapy, and prolonged estrogen exposure, increased density of breast tissue.
I would also add exposure to environmental endocrine disruptors and imbalances in the intestinal microbiome influencing estrogen metabolism.
Breast density and bone density are related to endogenous and exogenous estrogen exposure in a woman’s body. There is a correlation between estrogen exposure, high breast density, high bone density, and increased risk of breast cancer.
Bone is living metabolically active tissue. “Bone remodeling is the process by which bone is renewed to maintain bone strength and mineral homeostasis. Remodeling involves continuous removal of discrete packets of old bone, replacement of these packets with newly synthesized proteinaceous matrix, and subsequent mineralization of the matrix to form new bone begins before birth and continues until death. Bone remodeling increases in perimenopausal and early postmenopausal women and then slows with further aging, but continues at a faster rate than in premenopausal women. Bone remodeling is thought to increase mildly in aging men.” Normal Bone Anatomy and Physiology 10.2215/CJN.04151206
Engaging in a health model for all patients includes assessing and managing bone health to promote healthy bone over the life cycle. A health model for cancer patients, due to the typically older age demographics will inherently include a large population of patients already at risk for loss of bone mass, osteopenia and osteoporosis. Screening for bone mineral density and managing bone health should be part of whole-person, whole health care. Taking a thorough history that includes family history, bone health and bone mineral density can bring attention to patients at higher risk for low bone density and fracture as well as patients with a higher risk of estrogen driven breast cancers.
Bone density measurement is used in clinical medicine as an indirect indicator of osteoporosis and fracture risk. There is a clear association between poor bone density and higher probability of fracture. There is a clear association between poor bone density and low estrogen levels. Conversely there is a clear association between increased and healthy bone density and higher estrogen levels.
The Most Common Risk Factors for Low Bone Density and Primary Considerations for a Bone Density Test include:
- Females age 65 or older
- Males aged 70 or older
- People over age 50 with
- previous bone fracture from minor trauma
- rheumatoid arthritis
- low body weight
- a parent with a hip fracture
- Individuals with vertebral abnormalities
- Individuals receiving, or planning to receive, long-term glucocorticoid therapy
- Individuals with primary hyperparathyroidism
- Individuals being monitored to assess the response or efficacy of an approved osteoporosis drug therapy
- Individuals receiving androgen deprivation therapy
- Individuals with a history of eating disorders
Additional factors that are related to the risk of low bone density and the need for assessment include smoking, alcohol intake, long-term use of corticosteroid drugs, sedentary or convalescent lifestyle, protein status, mineral status, digestion, and absorption function, chronic inflammation and vitamin D status.
For cancer patients and survivors also consider periods of poor nutrition, calorie, protein status, convalescence, lack of exercise, effect of hormonal therapies, oophorectomy, orchiectomy, chemotherapy, immunotherapy, treatment induced thyroiditis, gastritis, enteritis and colitis, chronic pain impacting appetite, digestive and absorptive dysfunction, surgical loss of gastrointestinal organs and function as contributors to risk of loss of bone density and as well as multiple and varied adverse effects of cancer physiology and cancer treatments upon nutritional status and active lifestyle.
Selected References
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008 Nov;3 Suppl 3(Suppl 3):S131-9. doi: 10.2215/CJN.04151206. PMID: 18988698; PMCID: PMC3152283.
Fraenkel M, Novack V, Mizrakli Y, Koretz M, Siris E, Norton L, Shafat T, Geffen DB. Bone mineral density in women newly diagnosed with breast cancer: a prospective cohort study. NPJ Breast Cancer. 2022 Feb 17;8(1):21. doi: 10.1038/s41523-022-00388-z. PMID: 35177701; PMCID: PMC8854387.
Zain NM, Seriramulu VP, Chelliah KK. Bone Mineral Density and Breast Cancer Risk Factors among Premenopausal and Postmenopausal Women A Systematic Review. Asian Pac J Cancer Prev. 2016;17(7):3229-34. PMID: 27509955.